- Home
- Sixth Form
- Curriculum
- Chemistry
Chemistry
Introduction
Doing an A-level in chemistry can open many doors for you in the future. It is always seen as a challenging, academic and rigorous A-level that will impress a lot of universities and employers, therefore a qualification in chemistry opens doors to a wide range of career opportunities.
As well as practical knowledge of the subject, chemistry students develop many other useful skills, such as researching, problem solving, organisational skills and analytical skills. Group projects and presentations will also help develop your teamwork and communication skills.
The course is designed to lead to higher education; any degree course involving a scientific base will accept chemistry as an A Level.
Chemistry is perceived as an extremely versatile subject and is well respected by colleges and universities alike. Chemistry graduates also tend to be well paid! Recent research by PriceWaterhouseCoopers has shown that, on average, chemistry graduates earn more during their lifetimes than graduates of many other disciplines - up to 15 per cent higher in some cases!
Chemical scientists have a higher employment rate than students of other subjects. Over 70 per cent of chemistry students will enter a professional or managerial role when they have finished their studies and double the UK average go on to further study
Did you know...?
In 2018, the Nobel Prize for chemistry was awarded to a woman for only the fifth time in history! Frances H Arnold earned the award through her work on enzymes. The award was also awarded to George P Smith and Sir Gregory P Winter, for their work on peptides and antibodies. Could this be you one day?
The first Briton in space, Helen Sharman, and the first female British Prime Minister, Margaret Thatcher, both studied chemistry at university.
Course Description
We follow the EdExcel A Level Chemistry course (9CH0)
https://qualifications.pearson.com/en/qualifications/edexcel-a-levels/chemistry-2015.html
The two-year qualification combines scientific theory and practical skills relevant to understanding organic, inorganic and physical chemistry.
This qualification is intended to provide students with a great depth and breadth of understanding in order to challenge and stretch. As a student you will recognise the usefulness and limitations of a scientific method and appreciate its applicability in other disciplines and in everyday life.
Learning activities will include practical work, independent research, class discussions, presentations, online interactive tutorials and note taking.
Throughout the course you will develop the following skills:
- Using apparatus skilfully and safely
- Producing and recording valid and reliable measurements and observations
- Presenting and analysing data
- Research skills
- Identifying and evaluating resources
- Clarity of oral and written expression using scientific language
- Discussion and presentation skills
- Making notes
- Problem solving
The course is made up of the following topics:
| Topic 1 Atomic structure and the Periodic table
Topic 2 Chemical bonding and structure Topic 3 Redox reactions Topic 4 Inorganic chemistry and the Periodic table Topic 5 Formulae, equations and amounts of substances Topic 6 Organic chemistry Topic 7 Modern analytical techniques Topic 8 Chemical energetics Topic 9 Reaction kinetics
|
Topic 10 Chemical equilibrium
Topic 11 Further equilibrium Topic 12 Acid-base equilibria Topic 13 Further energetics Topic 14 Further redox Topic 15 Transition metals Topic 16 Further kinetics Topic 17 Further organic chemistry Topic 18 Modern Analytical Techniques
|
How will I be assessed?
Assessment is through three written papers taken at the end of two years of study.
|
Paper 1 - 90 marks, 105 mins (30%) |
Paper 2 - 90 marks, 105 mins (30%) |
Paper 3 - 120 marks, 150 mins (40%) |
|
Advanced Inorganic and Physical Chemistry |
Advanced Organic and Physical Chemistry |
General and Practical Principles in Chemistry |
|
Topic 1 Atomic structure and the Periodic table
Topic 2 Chemical bonding and structure Topic 3 Redox reactions Topic 4 Inorganic chemistry & the Periodic table Topic 5 Formulae, equations & amounts of substances Topic 8 Chemical energetics Topic 10 Chemical equilibrium Topic 11 Further equilibrium Topic 12 Acid-base equilibria Topic 13 Further energetics Topic 14 Further redox Topic 15 Transition metals |
Topic 2 Chemical bonding and structure
Topic 3 Redox reactions Topic 5 Formulae, equations & amounts of substances Topic 6 Organic chemistry Topic 7 Modern analytical techniques Topic 9 Reaction kinetics Topic 16 Further kinetics Topic 17 Further organic chemistry Topic 18 Modern Analytical Techniques |
All 18 topics of the course, including questions about practical techniques and analysis from the core practical tasks. |
Core practical tasks:
| Core Practical | Practical Techniques |
| 1: Measuring the molar volume of a gas | 1,4,11 |
|
2: Preparation of a standard solution from a solid acid |
1,4,5,6,11 |
|
3: Finding the concentration of a solution of hydrochloric acid |
1,4,5,6,11 |
|
4: Investigation of the rates of hydrolysis of halogenoalkanes |
1,2,4,11 |
| 5: The oxidation of ethanol | 2,4,7,11 |
|
6: Chlorination of 2-methylpropan-2-ol with concentrated hydrochloric acid |
1,4,7,8,9,11 |
|
7: Analysis of some inorganic and organic unknowns |
2,4,11 |
|
8: To determine the enthalpy change of a reaction using Hess’s Law |
1,4,11 |
| 9: Finding the Ka value for a weak acid | 3,4,11 |
| 10: Investigating some electrochemical cells | 4,10,11 |
| 11: Redox titration | 1,4,5,11 |
| 12: The preparation of a transition metal complex | 1,4,7,9,11 |
|
13a and 13b: Following the rate of the iodine- propanone reaction by a titrimetric method and investigating a ‘clock reaction’ (Harcourt-Esson, iodine clock) |
1,4,6,11,12 |
| 14: Finding the activation energy of a reaction | 1,4,11 |
| 15: Analysis of some inorganic and organic unknowns | 2,4,8,11 |
| 16: The preparation of aspirin | 1,2,4,7,8,9,11 |
Methods of Delivery
Students will have 5 hours per week in a specialised laboratory, where the key concepts of the course will be taught. During these lessons, students will also complete core practical tasks which will be internally assessed and endorsed by the Academy.
In addition to lessons, students will be required to complete at least three hours a week of independent study during their non-contact periods in their timetable. This is in addition to the weekly two hours of homework set by teachers.
Homework Expectations
In addition to lesson time students should spend at least 6 hours per week revising, practising questions, doing extra research and working independently outside of lesson time on their topics.
Careers
Future Careers
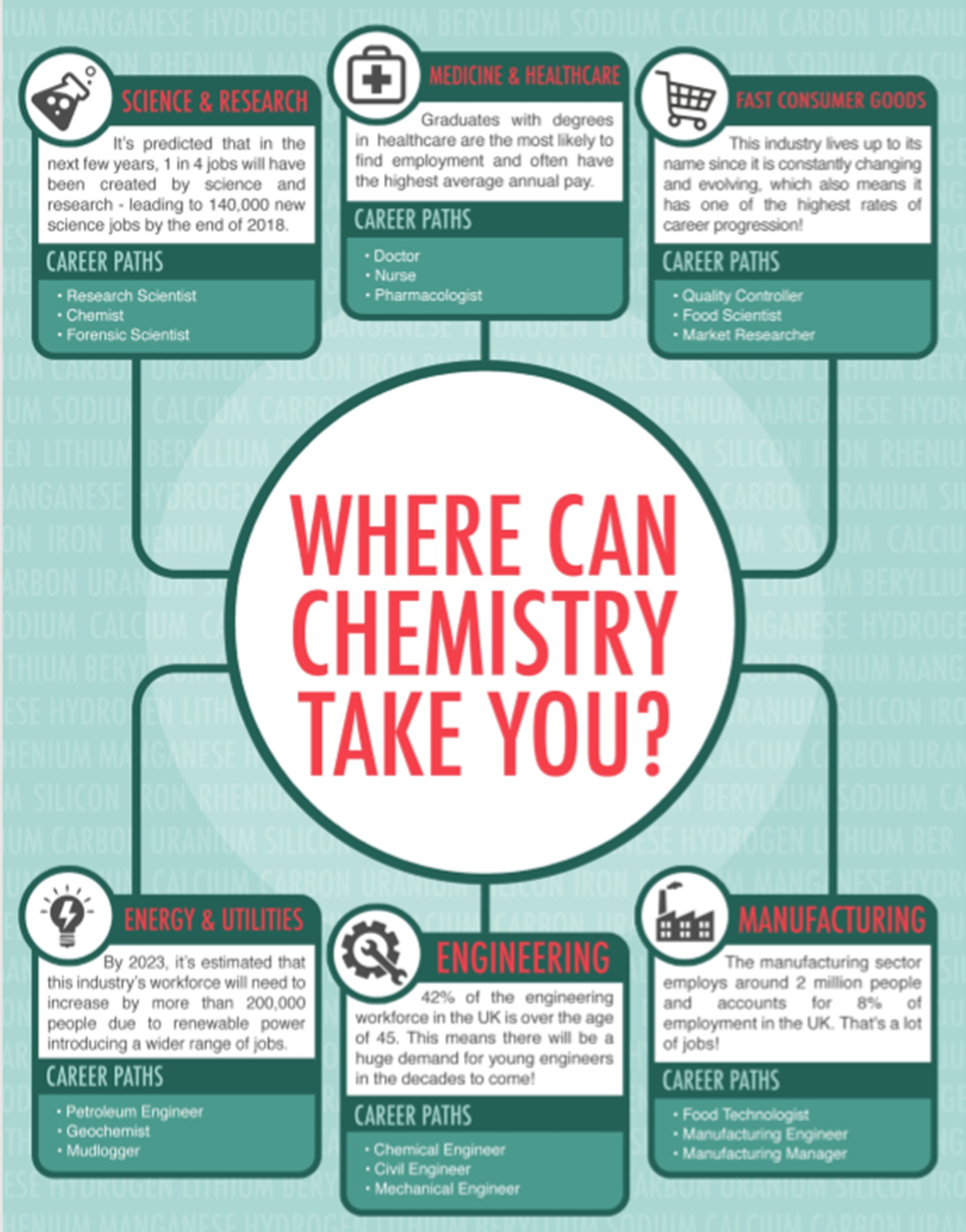
Chemistry will open the doors to countless careers, both in the lab and out of it, and people who have studied chemistry are employed in many sectors, including medicine research, manufacturing, and education. It is quite rightly seen as a challenging, academic and rigorous A level that will impress a lot of universities/employers.
A qualification in the chemical sciences opens doors to a wide range of career opportunities.
As well as practical knowledge of the subject, chemical science students develop many other useful skills, such as:
- problem solving; numeracy; data analysis making them good accountants, bankers and stockbrokers
- communication; research skills making them great solicitors and barristers, journalists and press officers
- creativity; team working; time management which are vital and highly valued skills in all employment.
Chemistry is also taken by many law applicants as it shows you can cope with difficult concepts.
Chemistry brings a nice balance to your studies if you are doing many Arts subjects. You need chemistry to study veterinary medicine. Almost every medical school in the country asks for A Level chemistry.
Doing A level chemistry can also lead to careers in healthcare such as medicine, pharmacy and dentistry but is also extremely useful in careers in the biological sciences, forensics, physics, mathematics, pharmacology and analytical chemistry.
Key Textbooks
Edexcel AS/A level Chemistry Student Book 1. ISBN: 9781447991168
Edexcel A level Chemistry Student Book 2. ISBN: 9781447991175
REVISE Edexcel AS/A Level Chemistry Revision Workbook. ISBN: 9781447989943
REVISE Edexcel AS/A Level Chemistry Revision Guide. ISBN: 9781447989974
Useful Links
Seneca Learning – www.senecalearning.com
Khan Academy - www.khanacademy.org/science/chemistry
Chemistry Guide – www.chemguide.co.uk
Doc Brown’s Chemistry Notes - http://www.docbrown.info/
Further Reading and Watching
BBC FOUR Documentary – Chemistry: A volatile history https://www.bbc.co.uk/programmes/b00qbq7f
Visual Curriculum Map
Chemistry



